Children of doctors will often tell you that their childhood complaints of aches and pains were almost always met with a healthy dose of skepticism from their parents. “You’ll be fine,” was the refrain. They were usually right. And so, last winter, when a sharp pain knifed through the inside of my hip while tying my shoe, I, at first, dismissed it, as I was taught to do. Denial persisted until one day, after working on skis all day, I had to ask a work colleague to unbuckle my boots for me. I couldn’t get to the buckles, and driving home in ski boots didn’t seem like a great idea.
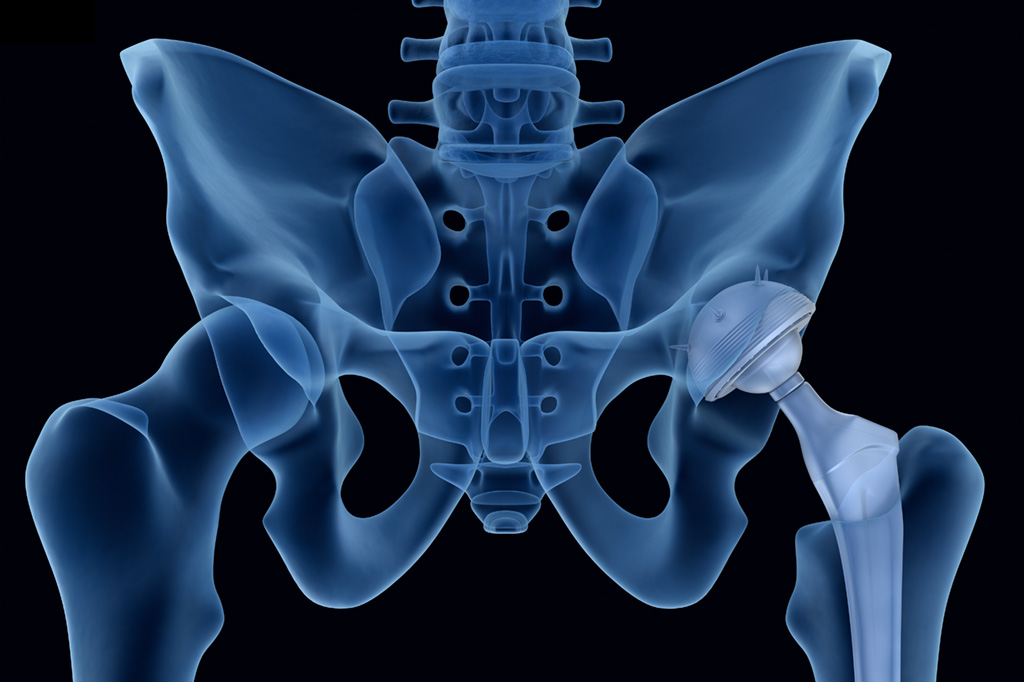
Not long after that I went to see Matt Kopplin, an orthopedic surgeon at St. Luke’s Wood River Medical Center in Ketchum. On an X-ray of my hip, he showed me how the gap between the ball of the ball-and-socket joint (the femoral head) and the receiving socket of the joint (the acetabulum) was gone. That gap, in a normal hip, houses cartilage, a sort of semi-solid lubricant between bones. The games we play—in my case, tennis, soccer, lacrosse, and skiing—wear away at cartilage over time, and, ultimately, result in bone articulating against bone. Hence, the knife-like pain.
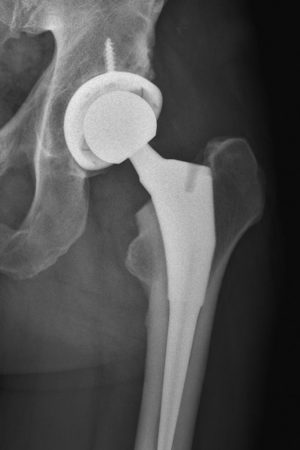
An X-ray image taken after hip replacement surgery shows a titanium shaft with a ceramic head (ball) inserted into the femur. A titanium and crosslinked polyethylene socket forms the receiving end of the artificial joint.
The solution to the problem—total hip replacement, formally termed total hip arthroplasty (THA)—involves removing these natural components evolved over 200,000 years and replacing them with marvels of precision-machined titanium, alumina, and crosslinked polyethylene, all refined over the last 58 years or so.
It turns out, I’m far from alone. In a 2015 Centers for Disease Control and Prevention report, researchers Monica Wolford, Kathleen Palso, and Anita Bercovitz determined that there were 310,800 total hip replacements in 2010. That was more than double the number that were done just 10 years prior. In addition, the age distribution of THA patients had shifted significantly toward younger patients: the percentage of patients in age groups 45 to 54 and 55 to 64 increased 5 percent each, while the percentage of patients in age groups 65 to 74 and 75 and over decreased by about 4 percent each. And a Mayo Clinic study presented in 2014 reported that there were approximately 2.5 million Americans walking around with artificial hips.
Hip replacement surgery really begins with an X-ray, which Kopplin will use along with a sophisticated software program to precisely size the parts needed and determine the positioning of them. In simplest terms, the surgery involves inserting a titanium stem with a ceramic ball on its end into the femur, which is essentially hollow, and then securing a titanium socket with a polyethylene liner—resembling two nested bowls—into the existing hip socket. All these parts exist in a dizzying array of sizes, as it is critical that the two legs end up being the same length.
As with the pre-surgery plan, the anesthesia plan is carefully choreographed with the end goal of enabling a patient to get up and move as soon as possible after surgery, often that same day. That plan, according to Kopplin, comprises three components—local anesthesia applied to the spinal fluid, a nerve block in the hip area, and anesthesia applied directly on the surgical site. These are all timed to come on board sequentially and can be titrated, so, as Kopplin explained, the patient “… doesn’t get run over by pain and have to play catch up by adding a lot of morphine,” which itself introduces problematic side effects.
There are two basic ways to get to the hip joint: from the front (anterior), or from the back (posterior), and surgeons tend to specialize in one more than the other.
“It is quite clear that if you get everything in the right place, hip patients do well with any exposure [posterior or anterior],” Kopplin said. However, he prefers to use the anterior approach, which does not involve cutting through muscles, but rather between them. That fact allows patients to get up out of bed sooner. The approach also allows him to do real-time X-rays during surgery to make sure the parts are exactly where he wants them to be.
The mechanics of the surgery are probably a little rough to stomach for the average reader, but, basically, they involve making an incision between muscle groups, removing the existing femoral head, hollowing out the femur, preparing the socket site, and then inserting the parts. The artificial socket is attached to the existing socket with a screw; the stem and ball are essentially press fit. “Bone loves titanium,” Kopplin said. Within six weeks, bone cells begin to grow into the porous titanium surface, and after a year the bond between the two is mature, he explained. There are no major nerves involved in the operation. As Kopplin said with a smile, “Orthopedic procedures are designed to stay as far away from nerves as possible.”
The patient experience now is quite different from what it was 20 years ago. This is largely due to the aggressiveness of mobilization—getting people up out of bed the day of surgery and walking shortly thereafter. This has improved outcomes and is primarily a function of physicians developing less invasive surgical techniques and better pain management protocols.
Technology has also played a part in improving outcomes. Particularly notable is the improved toughness of the polymeric socket. Not only is the polyethylene cross-linked with chemical bonds to add toughness, but also it is impregnated with Vitamin E to slow oxidation, a degradation process that many materials suffer in the presence of oxygen (think of, for example, iron parts that rust). What’s more, the improved toughness enables a surgeon to use a proportionately larger ball for the socket, which, in turn, reduces the risk of dislocation and makes for a more stable hip. Historically, say in the 1980s, Kopplin said, the rate of hip dislocations in THA patients might be between 3 and 5 percent. Now, it is 1 percent or less. In addition, the ceramic material used for the artificial femoral head is smoother than metal balls (cobalt-chromium) used previously. Smoother means less friction, which translates into less wear.
Almost immediately after hip replacement surgery, one begins physical therapy, a critical step in achieving a good outcome. While the therapy is not particularly grueling, it can be humbling. For me, that humbling came weekly at the hands of Jeni Cook, a physical therapist at St. Luke’s Rehabilitation. Cook had a remarkable ability to uncover physical motions and feats that I could not do. It was as if the surgery had scrambled the part of my brain managing my strength, balance, and coordination. Physical therapy felt like a process of rewiring it all.
As Cook explained, “The basic goal for physical therapy is to help people return to their desired level of function and activities after surgery … As a generalization, the primary focus is on ensuring that people regain their strength, range of motion, balance and proprioception (the perception of movement and spatial orientation), and normal gait pattern.”
The regimen focuses on developing core and hip strength, both of which stabilize the hip joint. Cook advised: “Get strong; stay strong … from your core through your feet!” However, there is also an emphasis on proper mechanics. As I discovered, with chronic pain type injuries, one can develop all kinds of convoluted coping mechanisms and bad habits to deal with a problem. Physical therapists like Cook can spot these subtle habits in a flash. Part of rehabilitation involves overcoming them.
So, in the end, the whole process was a relatively easy one: very little pain, a quick healing period, and a great outcome. Physically, I feel like I can do anything; if there are limits to that, I haven’t found them yet. Psychologically, I have to say it is a little odd to look at that X-ray and see those lifeless parts inside. What does all of that titanium and alumina have to do with me? And yet, it is me, now.
The question remains: how in the world does a human body take all of that inert metal and ceramic and turn it into something personal, something capable of running towards a child, turning a ski, shooting a soccer ball? It almost seems as if magic is involved.